Abstract
Background:
Early during the COVID-19 pandemic patient and provider anxiety concerning in-person visits and travel restrictions may have delayed cancer diagnosis and altered treatment. We evaluated changes in clinical presentation and treatment patterns in multiple myeloma (MM) during the early COVID-19 period compared to historical pre-COVID periods.
Methods: Using the nationwide Flatiron Health EHR-derived de-identified database, we compared clinical presentation and treatment patterns in the immediate post-COVID period (2020) to a comparable pre-COVID period (2018 and 2019). We focused on two separate clinical settings: 1) patients newly diagnosed with MM during February-June in the years of interest (NEWPT) with evidence of management within 90 days and follow-up for 7 months; and 2) patients diagnosed with MM during 2014-2019 receiving active treatment as of February (2018, 2019, 2020, ACTIVE) and follow-up for 11 months. Delayed clinical presentation was assessed using baseline (90 days before diagnosis/index date) measures of ISS stage, ECOG performance status, anemia, and kidney function. We examined treatment patterns (choice of regimen) of both cohorts in the two time periods. We compared clinical features of initial presentation in pre-COVID and COVID period using Pearson's χ 2 test. For NEWPT, we also utilized Kaplan-Meier curves and log-rank test to compare time to treatment initiation between the two periods. Multivariable Cox proportional hazards regression model with death as a competing risk was used to determine impact of COVID on treatment initiation by adjusting sex, age at diagnosis, race, insurance, stage, baseline ECOG, and hospital setting. All analyses were conducted in SAS (Version 9.4, SAS Institute, Cary, North Carolina) with 2 sided tests and a type I error of 5%.
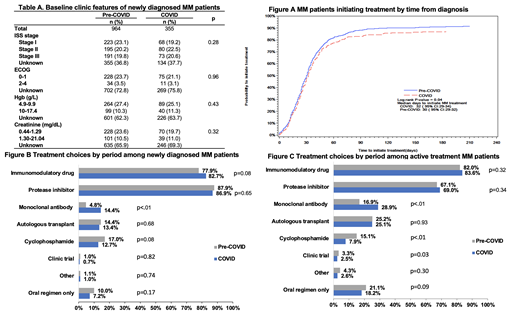
Results: Our study included 1319 NEWPT (964 pre-COVID and 355 COVID) and 2206 ACTIVE (1014 pre-COVID and 1192 COVID) patients. In the NEWPT cohort, we observed no differences between the pre-COVID and COVID periods in terms of baseline characteristics, including clinical features like stage, ECOG performance status, anemia or kidney function (Table A). Patients in the pre-COVID period were more likely to initiate any treatment (91.1% vs 86.2%, p<.01). Median time to treatment initiation was 30 days pre-Covid and 32 days during the Covid period (log-rank test p=0.04, Figure A). After adjusting for patient demographic, clinical features (extent of anemia, hypercalcemia, kidney dysfunction), and hospital variables (US region, practice type academic vs community), the difference between the two periods was not significant (COVID vs pre-COVID hazard ratio=0.88, 95% confidence interval 0.78-1.10, p=0.07). In NEWPT cohort, compared with their pre-COVID counterparts, patients in COVID period were more likely to receive monoclonal antibody (mAb) (14.4% vs 4.8%, p<.01, Figure B) and used IMID-based regimen as their first line of therapy (80.4% vs 74.3%, p<.01).
In ACTIVE cohort, more patients in the pre-COVID period were anemic (Hemoglobin <10 g/L, 14.9% vs 9.7%, p<.01) at baseline than those in the COVID period. As in NEWPT cohort, ACTIVE patients in the COVID period used mAb-based regimen more commonly (28.9% vs 16.9%, p<.01) (Figure C). In addition, fewer ACTIVE treatment patients in the COVID period received cyclophosphamide regimens (7.9% vs 15.1% p<.01).
Conclusions: During early COVID-19 pandemic we did not observe evidence of delayed diagnosis or more advanced stage, anemia or kidney disease for NEWPT with MM. MM treatment patterns were notable for higher utilization of mAb, IMID-based therapies and decreased use of cyclophosphamide regimens, without significant change in time to treatment initiation. Reassuringly, changes in treatment-patterns during COVID pandemic were modest, some likely reflecting changes in MM treatment landscape (advances in mAb regimens) rather than direct impact of COVID. Further studies are needed to understand how these changes evolve and affect clinical outcomes over time beyond 2020.
Neparidze: GlaxoSmithKline: Research Funding; Eidos Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Research Funding. Zeidan: Jasper: Consultancy; AstraZeneca: Consultancy; Aprea: Consultancy, Research Funding; Gilead: Consultancy, Other: Clinical Trial Committees; Loxo Oncology: Consultancy, Other: Clinical Trial Committees; Astellas: Consultancy; Agios: Consultancy; Kura: Consultancy, Other: Clinical Trial Committees; Jazz: Consultancy; Pfizer: Other: Travel support, Research Funding; Genentech: Consultancy; Geron: Other: Clinical Trial Committees; BMS: Consultancy, Other: Clinical Trial Committees, Research Funding; ADC Therapeutics: Research Funding; Novartis: Consultancy, Other: Clinical Trial Committees, Travel support, Research Funding; Boehringer Ingelheim: Consultancy, Research Funding; Astex: Research Funding; BeyondSpring: Consultancy; Incyte: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Epizyme: Consultancy; BioCryst: Other: Clinical Trial Committees; Cardiff Oncology: Consultancy, Other: Travel support, Research Funding; Janssen: Consultancy; Ionis: Consultancy; Amgen: Consultancy, Research Funding; Acceleron: Consultancy, Research Funding; AbbVie: Consultancy, Other: Clinical Trial Committees, Research Funding. Podoltsev: Pfizer: Honoraria; PharmaEssentia: Honoraria; Blueprint Medicines: Honoraria; Incyte: Honoraria; Novartis: Honoraria; CTI BioPharma: Honoraria; Bristol-Myers Squib: Honoraria; Celgene: Honoraria. Shallis: Curis: Divested equity in a private or publicly-traded company in the past 24 months. Ma: Celgene/Bristol Myers Squibb: Consultancy, Research Funding. Davidoff: Amgen: Consultancy; AbbVie: Other: Family member consultancy. Huntington: AstraZeneca: Consultancy, Honoraria; TG Therapeutics: Research Funding; Thyme Inc: Consultancy; Flatiron Health Inc.: Consultancy; Genentech: Consultancy; SeaGen: Consultancy; Novartis: Consultancy; Pharmacyclics: Consultancy, Honoraria; Servier: Consultancy; Bayer: Honoraria; DTRM Biopharm: Research Funding; AbbVie: Consultancy; Celgene: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal